Eric Krukonis
Eric Krukonis
Associate Professor
Assistant Director of Research


Degrees
- Ph.D., Tufts University
Biography
Prof. Krukonis teaches oral microbiology and infection control to Dental Hygiene students, oral microbiology, infection control, and infectious diseases to Dental students and current topics in microbiology to Residents in Periodontology, Endodontics and Orthodontics.
Current areas of dental research include: the etiology of root caries in geriatric populations, infection control in the dental clinical, and characterizing antimicrobial properties of essential oil products.
In addition, the Krukonis laboratory studies the molecular mechanisms of bacterial pathogenesis by the medical pathogens Vibrio cholera (the causative agent of cholera) and Yersinia pestis (the causative agent of plague). Both are model organisms for the study of infectious disease. Some recent publications in these areas are listed below.
Dr. Krukonis got his BA in Biology from Rice University in 1990 and his PhD in Molecular Biology and Microbiology from Tufts University in 1998. He joined the faculty of Detroit Mercy Dental in 2013.
Research Activity
Current areas of dental research include: the etiology of root caries in geriatric populations, infection control in the dental clinical, and characterizing antimicrobial properties of essential oil products.
In addition, the Krukonis laboratory studies the molecular mechanisms of bacterial pathogenesis by the medical pathogens Vibrio cholera (the causative agent of cholera) and Yersinia pestis (the causative agent of plague). Both are model organisms for the study of infectious disease.
Complete list to published works: Prof. Eric Krukonis
-
Research Projects
1. Understanding the Microbiology of Root Caries in Geriatric Patients
As people age their gums often recede revealing root surfaces to bacterial colonization and caries development. In the past, a few acid-generating bacteria have been implicated in the etiology of root caries, but with modern molecular techniques the collection of bacteria shown to be associated with root caries is expanding. Using techniques of quantitative PCR and microbiome analysis on dental plaque from diseased and health root surfaces we are defining those bacteria that are more prevalent on carious root surfaces and investigating their potential contributions to root caries. Our long-term goal is to develop therapies that target root caries-causing bacteria for elimination from dental plaque.
2) Defining Pathogenic Mechanisms of the Human Pathogen Yersinia pestis, the Causative Agent of Plague
Plague is one of the most devastating diseases in human history and still exists in a number of regions in the world today. While only a few cases of plague are documented each year in the U.S., it is of concern as a potential bioterrorism threat. Our laboratory performs research to understand the mechanisms of pathogenesis of Y. pestis in hopes of finding vulnerabilities to be exploited in the design of anti-plague therapeutics. We focus on defining the mechanism of toxin delivery from Y. pestis to human cells, a step required for disease as well as understanding how Y. pestis survives in human blood during plague infection despite our normal antimicrobial killing capacity.
The Krukonis Laboratory uses Yersinia pestis, the causative agent of plague, as a model organism to study how bacterial pathogens delivery cytotoxins to host cells resulting in disruption of normal cellular functions including cytoskeletal arrangements and immune pathways. Y. pestis is shown in association with the human epithelial cell line HEp-2, where the cytoskeleton has collapsed and cells are rounded indicating cytotoxicity caused by injection of several cytotoxic proteins known as Yops.
3) Regulation of Virulence Gene Expression in Vibrio cholerae, the Causative Agent of Cholera
The disease cholera is a rapidly progressing diarrheal disease that causes >100,000 infections and thousands of deaths each year (World Health Organization data). Bacterial pathogens express different genes according to their environment so they tailor protein expression to the conditions in which they are growing. V. cholerae, senses when it is ingested by a human host by a change in temperature and other environmental signals to trigger a program of gene expression that allows for efficient colonization of the human host and expression of the key virulence factor, cholera toxin. Expression of cholera toxin results in up to 20 liters of diarrheal excretion per day in patients suffering from cholera leading to a life-threating dehydration and allowing rapid dissemination of V. cholerae back into the environment to infect additional hosts. Our laboratory studies the molecules utilized for initiating the gene expression program required to establish cholera toxin expression as well as proteins required for host colonization. Our hope is to identify chemical compounds that can inhibit this process and could be used as therapeutics during cholera outbreaks.
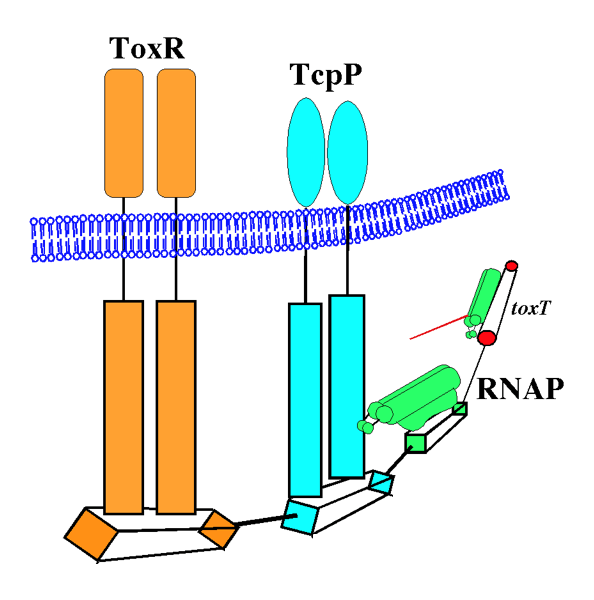
The Krukonis Laboratory investigates the mechanisms of virulence gene regulation, including regulation of cholerae toxin production in the diarrheal pathogen Vibrio cholerae by two membrane–localized transcription factors ToxR and TcpP. ToxR and TcpP activate the toxT promoter. The ToxT protein then directly activates cholera toxin and other genes required for colonization and virulence.
-
Grants & Funding
The Krukonis lab has a grant from NIH Institute of Allergy and Infectious Disease (NIAID)
NIH R21 AI 133570 entitled:
“Ail-mediated serum resistance in Yersinia pestis and its contribution to plague
virulence”
The Krukonis Lab has a grant from the University of Detroit Mercy School of Dentistry entitled:
“Explorations in Bacterial Pathogenesis”
In addition, the Krukonis lab participates in anotherDMSOD grant entitled:
“Isolation of bacteriophages that target and kill dental pathogens”
-
Publications
PUBLICATIONS
Some key publications from our laboratory are:
Krukonis, E.S. and Thomson, J.J. 2020 Complement Evasion Mechanisms of the
Systemic Pathogens Yersiniae and Salmonellae FEBS J. 594 (16) pp.2598-2620.
Invited Review
Morgan, S.J., French, E.L., Plecha, S.C., and Krukonis, E.S. 2019 The wing of the ToxR
winged helix-turn-helix domain is required for DNA binding and activation of toxT
and ompU PLoS One 14(9) e0221936
Thomson, J.J., Plecha S.C. and Krukonis E.S. 2019 Ail provides multiple mechanisms of
serum resistance to Yersinia pestis Mol Microbiol Vol 111 (1) pp. 82-95
Davidson, T., Lewandowski, E., Smerecki, M., Stratton, H., Alhabeil, J., Wheater, M.,
Shepherd, K. and Krukonis, E.S. 2017 Taking Your Work Home With You: Potential
Risks of Contaminated Clothing and Hair in the Dental Clinic and Attitudes about Infection Control Can J Inf Cntl Vol 32 (3) pp. 137-142.
Tsang, T. M., Wiese, J. S., Alhabeil, J. A., Usselman, L. D., Thomson, J. J., Matti, R.,
Kronshage, M., Maricic, N., Williams, S., Sleiman, N. H., Felek, S. and Krukonis, E.
- 2017. Defining the Ail Ligand-Binding Surface: Hydrophobic Residues in Two
Extracellular Loops Mediate Cell and Extracellular Matrix Binding To Facilitate Yop
Delivery. Infect Immun. Vol. 85 (4) e01047-15.
Morgan, S.J., French, E.L., Thomson, J.J., Seaborn C.P., Shively, C.A. and Krukonis,
E.S. 2015. Formation of an intramolecular periplasmic disulfide bond in TcpP protects TcpP and TcpH from degradation in Vibrio cholerae. J. Bact. Vol. 198(3) pp. 498-509.
Goss, T.J, Morgan, S.J., French, E.L. and Krukonis, E.S. 2013. ToxR recognizes a direct
repeat element in the toxT, ompU, ompT and ctxA promoters of Vibrio cholerae to
regulate transcription. Infect. Immun. Vol. 81(3) pp. 884-895.
Morgan, S.J., Felek, S., Gadwal, S., Koropatkin, N.M., Perry, W.J., Bryson, A.B., and
Krukonis, E.S. 2011. The two faces of ToxR: activator of ompU, co-regulator of
toxT in Vibrio cholerae, Mol. Micro. Vol. 81 (1) pp. 113-128.
Tsang, T. M., Felek, S. and Krukonis, E. S. 2010. Ail binding to fibronectin facilitates
Yersinia pestis binding to host cells and Yop delivery. Infect. Immun. Vol. 78 (8)
pp.3358-3368.
Goss, T. J., Seaborn, C. P., Gray, M. D. and Krukonis, E. S. 2010. Identification of the
TcpP-binding site in the toxT promoter of Vibrio cholerae and the role of ToxR in
TcpP-mediated activation. Infect. Immun. Vol. 78 (10) pp. 4122-4133.
Felek, S., Tsang, T. M. and Krukonis, E. S. 2010. Three Yersinia pestis adhesins
facilitate Yop delivery to eukaryotic cells and contribute to plague virulence. Infect.
Immun. Vol. 78 (10) pp. 4134-4150.
Felek, S. and Krukonis, E.S. 2009. The Yersinia pestis Ail protein mediates binding
and Yop delivery to host cells required for plague virulence. Infect. Immun., Vol.
77 (2) pp.825-836. Cover article
A Complete List of Published Works Can be Found in MyBibliography:
https://www.ncbi.nlm.nih.gov/myncbi/1dUqahXgeMW5R/bibliography/public/
-
Lab Members
Current Lab Members:
Dr. Sarah Plecha
Postdoctoral Fellow
Karmen Rucker
Laboratory Technician and Lab Coordinator
Laura Young
Laboratory Technician
Dental Students:
Lucas Mathes – DS4
Michele Bhagwagar – DS3
Jay Wayntraub – DS3
Christen Thompson – DS3
Eilkay Samadi – DS3
William Vo – DS3
Nik Tasevski – DS3
Colleen O’Brien – DS3
Periodontics Residents:
Dr. Jonathan Zora
Detroit Mercy Undergraduates:
Amber Abram - BUILD Scholar
Sean Ojha - Detroit Mercy Undergraduate
Past Lab Members:
Jamal Alhabeil, former Laboratory Technician and Lab Coordinator; currently teaching English Language in Nagahama, Japan for the Shiga Board of Education
Dr. Joshua Thomson
former Postdoctoral Fellow – currently Assistant Professor: University of Detroit Mercy School of Dentistry
Dr. Emily French
former laboratory technician – currently in the La Crosse-Mayo Family Medicine Residency Program in La Crosse, WI; MD from Central Michigan University
Jeffrey Wiese
former laboratory technician – currently a Senior Medical writer at MMS Holdings
Dr. Suleyman Felek
former Postdoctoral Fellow – currently practicing Internal Medicine at Richmond University Medical Center, Waterbury Hospital and Yale-New Haven Children's Hospital
Dr. Sarah Morgan
former PhD student – currently a Senior Postdoctoral fellow/lab manager at University of Washington in the laboratory of Dr. Pradeep Singh
Dr. Tiffany Tsang
former PhD student – currently a science contractor between the US government and private industry
Dr. Thomas Goss
former laboratory technician – currently a senior laboratory technician at the University of Michigan Medical School
Malte Kronshage MS
former Masters student from Germany
Former University of Detroit Mercy Undergraduates
Christina Jones - former BUILD Scholar: currently a DDS/PhD candidate at the University
of Michigan School of Dentistry, in the laboratory of Dr. Isabelle Lombaert
Nour El Yaman – former BUILD Scholar
Lizbeth Garcia-Leon – former BUILD Scholar
Former Dental Students:
Zachary Mayer DDS
Keeton Colville DDS
Mohamed El-Shaer DDS
Elizabeth Doman DDS
Sumita Sam DDS
Lisa Park DDS
Shameel Khan DDS
Michelle Szewczyk DDS
Ayah Koleilat DDS
Malaka Saleh DDS
Jonathon Zora DDS
Chelseas Watkins DDS
Former Hygiene students:
Hina Qadir RDH
Teniece Roberts RDH
Megan Patlow RDH
Terlicia Winston RDH
Alexandra Willaeys RDH
Taylor Davidson RDH
Meghan Smerecki RDH
Halee Stratton RDH
Erica Lewandowski RDH
Sarah Charland RDH
Alyson Fryz RDH
Sara Trombly RDH
Heather VanOast RDH
Rajpreet Grover RDH
Shamaila Mirza RDH
Navneet Somal RDH
Hannah Howard RDH
Vrushi Patel RDH
Mirna Yousif RDH
Amanda Totin RDH
Former Dental Residents:
Krupa Patel DDS
